Cu to Cu2+ Oxidation or Reduction
To solve this you need oxidation number rules search the internet. It does not ionize and hence do not conduct electricity.

Chapter 19 Electrochemistry Ppt Download
Reduction is a gain of electrons.
. Furthermore we find that electrochemical reduction waves observed in the CVs220 because the potentials oxidation of a Cu-related trap state into Cu2 reduces the are close to what has been measured in water for the CuCu2 PL intensity due to an efficient Auger recombination pathway couple48 We note that the CuCu2 reduction and. For example Cu oxidation state of the Cu modified TiO 2 84. Cu 2 aq Cus reduction Als Al 3 aq oxidation 2.
A The reaction takes place at anode. Copper is OXIDIZED from copper metal mathCu0math to cupric ion mathCu0math to cupric ion. Identify which reactants are being oxidized the oxidation number increases when it reacts and which are being reduced the oxidation number goes down.
The conversion of Cu2 to Cu indicates. B reduction by GAIN of electrons 2. Assign oxidation numbers to each species.
D reduction by LOSS of electrons 2. Fe s Fe 2 aq 2e- Oxidation half reaction Cu2 aq 2e- Cu s Reduction half reaction. Chemistry questions and answers.
In this Zn is changing from 0 to 2 and this is an oxidation while CuII is changing to Cu and going from 2 to 0. Oxidation number also called oxidation state is a measure of the degree of oxidation of an atom in a substance see. Some are more complex eg SO32- --- SO42-.
Cu Ag NO3- --- Ag Cu2 2NO3-Cu is being oxidized and Ag is being reduced. An increase in oxidation state is oxidation eg Zn and a decrease in oxidation state is reduction eg CuII. Similarly Co II was doped on TiO 2 by an impregnation method and H 2 production rate was enhanced under solar light irradiation.
The oxidation state decreases from 2 to zero 2 electrons gained. The copper is reduced. And identify what is being oxidized and what is being reduced.
When the full redox reaction is balanced the number of moles of electrons transferred in the reaction is. These two half reactions are given below. The pattern of LDL response to Cu2 observed in dialyzed plasma could be reproduced by adding 3 bovine serum albumin to isolated LDL.
A oxidation by GAIN of electrons 2. Cu sCu2 aqAg aqAg s Anode oxidation Cu s Cu2 aq 2e-Eo ox -Eo Cu -0337 v Cathode reduction Ag e- Ag s Eo red E o Ag 0800 v 2 x Ag e- Ag s 0800 v Cu s Cu2 aq 2e -0337 v Cu s 2Ag aq Cu2 aq 2 Ag s 0463 v Eo cell Or. At 250 microM Cu2 other evidence of LDL oxidation was found.
The two half-reactions are as follows. CISCE ICSE Class 9. C oxidation by LOSS of electrons 2.
Cu 2 2 I -1 - Cu 1 I 0 2 b Identify and write out all redox couples in reaction. When the fraction of the aggregated copper is high as. The oxidation process involves.
Cu 0 Cu 2 2. Question Bank Solutions 14659. We can write two half reactions for this process one for Fe to Fe2 and the other for Cu2 to Cu.
Cu2 An oxidation-reduction reaction is to be made from the following two reduction half reactions. In both species of enzyme rapid reduction of Cu2 ions by substrate takes place. At a higher Cu2 concentration 50 microM an increased expression of the Bsol7 epitope was observed.
The FORMAL LOSS of electrons specifies oxidation and we introduce oxidation numbers as labels to make electron lossgain specific. Note that the two-electron reduction lowers the oxidation state of copper from 2 in. Answer 1 of 19.
The added electrons reduce the oxidation state of the substance. 37 Cu2 undergoes the process of a. The colourless solution of silver nitrate slowly turns blue on adding copper chips to it because of A Dissolution of Copper B Oxidation of Ag Ag C Reduction of Cu2 ions D Oxidation of Cu atoms.
Oxidation of Cu atoms. Als Cu 2 aq Al 3 aq Cus 1. Cu2 2 E- Cu.
Concept Notes Videos 430. The hindrance of Cu2 reduction found after activation may reflect higher stability of Cu-0-Cu entities or stabilisation of the higher oxidation state by interaction with framework oxygen atoms. Please log in or register to add a comment.
OX Cu --- Cu2 RED Ag --- Ag. Professors will sometimes give you a mnemonic like LEO GER for loss of electrons is oxidation and gain of electrons. Standard Oxidation Potential SOP under standard conditions.
Advertisement Remove all ads. State Whether the Following Conversion is Oxidation Or Reduction. Cu Ag NO3- --- Ag Cu2 2NO3-Step 3.
As rightarrow Ac Ce- Coppers Standard Oxidation Potential Cu s rightarrow Cu2 2e- E_0o SOP -034 V The standard oxidation potential and the standard reduction potential are opposite in sign to each other for the same chemical species. Eo cell EoCathode - EoAnode right left reduction. C Carbon tetrachloride is a non-electrolyte because it is a covalent compound.
This is an oxidation-reduction reaction since the oxidation numbers changed. 2e Cu s O2 g 4H 4e 2H2O 1 To create a redox reaction one of these reactions will have to become an oxidation. B Cu2 will discharge easily at cathode.
This process will happen in the b. You are taking two electrons out of that neutral copper atom to make a copperII cation. Rules for assigning oxidation numbers.
The oxidation number of Xe in BaXeO6 is A 8 B 6 C 4 D 10. The oxidation state increases from zero to plus three 3 electrons lost. Strikingly it was found that the oxidation rate is 82 times faster at pH 74 for bilayers containing 70 mol PE than for pure phosphatidylcholine PC bilayers upon exposure of both to 70 μM Cu 2 and 10 mM hydrogen peroxide.
In the active enzyme Cu ions are reactive to O2 but in the inactive enzyme Cu can be oxidized only by oxidizing agents such as H2O2 or ferricyanide or by a slow intermolecular electron transfer from Cu 615 to the active enzyme. The oxidation of double bonds in PE-containing bilayers can be monitored in the presence of Cu 2. Cu2 ions gain two electrons so they are reduced to Cu atoms.
Pb Cu2 - Pb2 Cu Pb undergoes a process called. This is an example of oxidation. State Whether the Following Conversion is Oxidation Or Reduction.

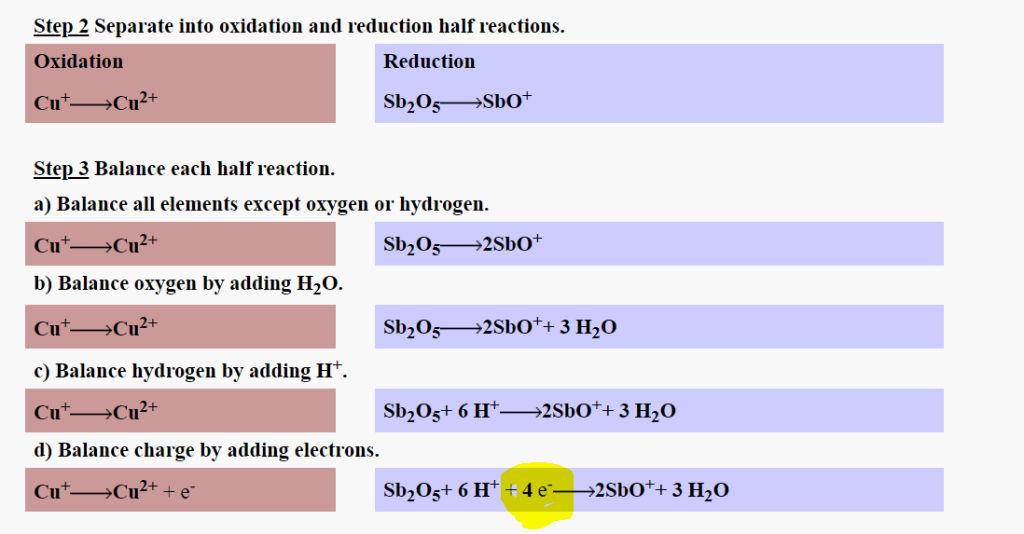
Solved Step 2 Separate Into Oxidation And Reduction Half Chegg Com

Electrochemistry Experiment 12 Oxidation Reduction Reactions Consider The Reaction Of Copper Wire And Agno 3 Aq Agno 3 Aq Ag S Cu S Ppt Download

Comments
Post a Comment